
AS is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts, and is supported by Award Number R01 AI122232 and start-up funds from Award Number P20RR021905 from the National Institute of Allergy and Infectious Disease and the National Institute of General Medical Sciences, respectively. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are creditedĭata Availability: All relevant data are within the paper and its Supporting Information files.įunding: KAF is supported by T32 AI055402 from the National Institute of Allergy and Infectious Disease. Received: FebruAccepted: SeptemPublished: October 14, 2015Ĭopyright: © 2015 Fimlaid et al.

Kearns, Indiana University, UNITED STATES difficile spore formation and thus disease transmission.Ĭitation: Fimlaid KA, Jensen O, Donnelly ML, Siegrist MS, Shen A (2015) Regulation of Clostridium difficile Spore Formation by the SpoIIQ and SpoIIIA Proteins. The results of our study suggest that targeting these conserved proteins could prevent C. difficile are dispensable for forespore transcription, although they are important for completing forespore engulfment and retaining the protective spore coat around the forespore, in contrast with B.

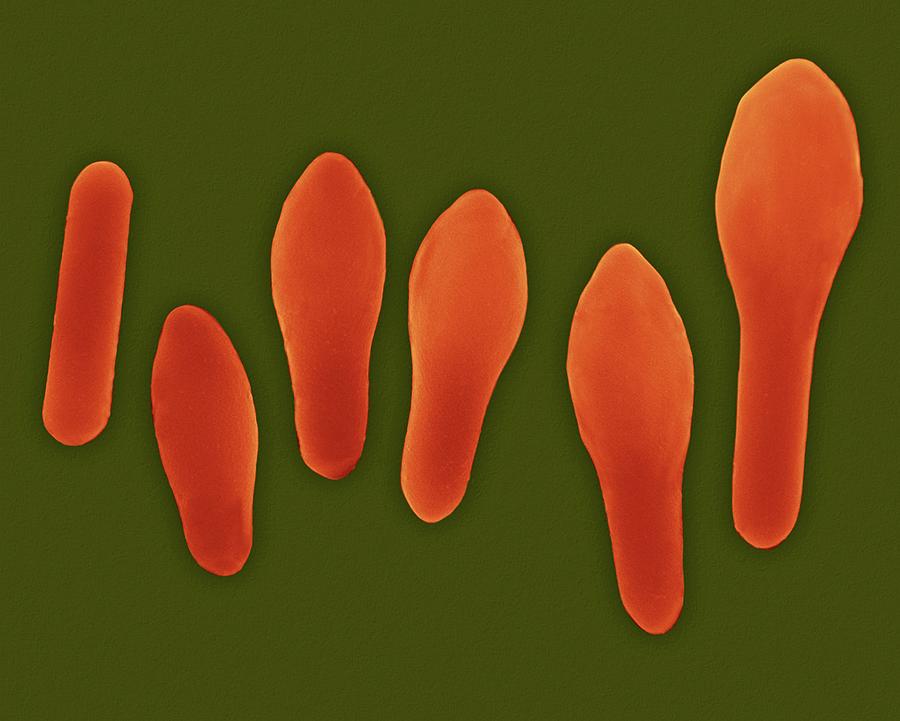
We show here that conserved components of this structure in C. In the model organism Bacillus subtilis, this channel had previously been shown to function as a “feeding tube” that allows the mother cell to nurture the developing forespore and sustain transcription in the forespore. Since the mechanisms controlling this process remain poorly characterized, we analyzed the importance of highly conserved secretion channel components during C. Accordingly, spore formation is essential for C. difficile must form spores in order to survive exit from the gastrointestinal tract. The bacterial spore-forming pathogen Clostridium difficile is a leading cause of nosocomial infections in the United States and represents a significant threat to healthcare systems around the world. difficile spores and thus disease transmission. Taken together, our findings suggest that inhibiting SpoIIQ, SpoIIIAA, or SpoIIIAH function could prevent the formation of infectious C. subtilis, which requires both Walker A and B motifs for SpoIIIAA function. Interestingly, mutation of the conserved Walker A ATP binding motif, but not the Walker B ATP hydrolysis motif, disrupted SpoIIIAA function during C. difficile SpoIIQ directly interacts with SpoIIIAH. In vitro pull-down assays further demonstrated that C. Although the spoIIQ, spoIIIA, and spoIIIAH mutants were defective in engulfment, metabolic labeling studies revealed that they nevertheless actively transformed the peptidoglycan at the leading edge of engulfment. difficile spoIIQ, spoIIIA, and spoIIIAH mutants exhibited defects in engulfment, tethering of coat to the forespore, and heat-resistant spore formation, even though they activate σ G at wildtype levels. In this study, we investigated the requirements of the SpoIIQ and SpoIIIA proteins during C. Even though these channel components are conserved in all spore formers, recent studies in the major nosocomial pathogen Clostridium difficile suggested that these components are dispensable for σ G activity. σ G activity in the forespore depends on the formation of a secretion complex, known as the “feeding tube,” that bridges the mother cell and forespore and maintains forespore integrity. In the model organism Bacillus subtilis, sporulation-specific sigma factors activate compartment-specific transcriptional programs that drive spore morphogenesis. Sporulation is an ancient developmental process that involves the formation of a highly resistant endospore within a larger mother cell.


 0 kommentar(er)
0 kommentar(er)
